Annutrofil: preserving the health of your hair.
Annurtrofil® is an extract of Annurca apple(Malus pumila Miller cv Annurca). It helps maintain healthy skin. It also strengthens hair by thickening it, increasing density and reducing hair loss.
Thank you for your request.
We’ll get back to you as soon as possible!
Continue on page View our catalogWhy choose Annurtrofil©?

What is Annutrofil©?
Annutrofil is an extract of the Annurca apple(Malus pumila Miller cv Annurca), or melannurca, an apple variety from the Campania region of southern Italy with PGI (Protected Geographical Indication) status. The aim of Annurtrofil® is to add value to an extract of this apple variety, whose properties for hair health have been studied. This in vitro study 1 demonstrates the activity of Annurca extract in regulating the expression of hair keratin, promoting hair thickening and density, and reducing hair loss.
1. Piccolo M, Ferraro MG, Maione F, Maisto M, Stornaiuolo M, Tenore GC, Santamaria R, Irace C, Novellino E. I Nutrients. 2019 Dec 13;11(12):3041.

A solid partnership, a guarantee of trust.
For over 15 years, our partner EVRA has operated an extraction unit in southern Italy (Pollino Park), with the aim of enhancing the value of plant species endemic to this region. The region is under the influence of the Mediterranean, Ionian and Adriatic seas, providing a unique microclimate for the cultivation of medicinal and perfume plants.

A unique harvesting and extraction process.
As part of a short circuit with producers, collection commitment contracts are drawn up. The collection supply chain is simple and straightforward. After being harvested unripe, the apples are placed on structures to complete their ripening: the entire surface of the apples is exposed to the sun. The apples are then continuously turned by hand until the skin has taken on the uniform intense red color of Annurca apples. This process promotes the formation and concentration of Proacyanidins B2, which are known to increase the keratin content of hair 2. This apple variety is also rich in gallic acid. Finally, the extraction process was developed to be traditional, i.e. without organic solvents.
2. Badolati N, Sommella E, Riccio G, Salviati E, Heintz D, Bottone S, Di Cicco E, Dentice M, Tenore G, Campiglia P, Stornaiuolo M, Novellino E. Nutrients. 2018 Oct 2;10(10):1406.

Annurtrofil® clinicalstudy
The aim of Study 3 was to explore the tolerability and clinical efficacy of oral Annurtrofil® supplementation on hair health. The study was carried out on 80 subjects aged between 18 and 60, evenly distributed with 50% men and 50% women. The study was double-blind and randomized. In other words, random assignment to two distinct groups:
- The control group (placebo), comprising 40 subjects, took 2 capsules/d corresponding to a daily dose of 800 mg maltodextrin.
- Experimental group with 40 subjects taking 2 capsules/d, i.e. 800 mg Annurtrofil®.
Evaluation took place over several time frames: from inclusion at T0, at T60, at T120 and until the end of supplementation at T180, then after 30 days of follow-up. Capillary density and thickness were measured using optical profilometry.
3. De Biasio, F et al, Journal of Applied Cosmetology. 41, 2 (May 2023), 28/46 https://doi.org/10.56609/jac.v41i2.276
Efficacy study results
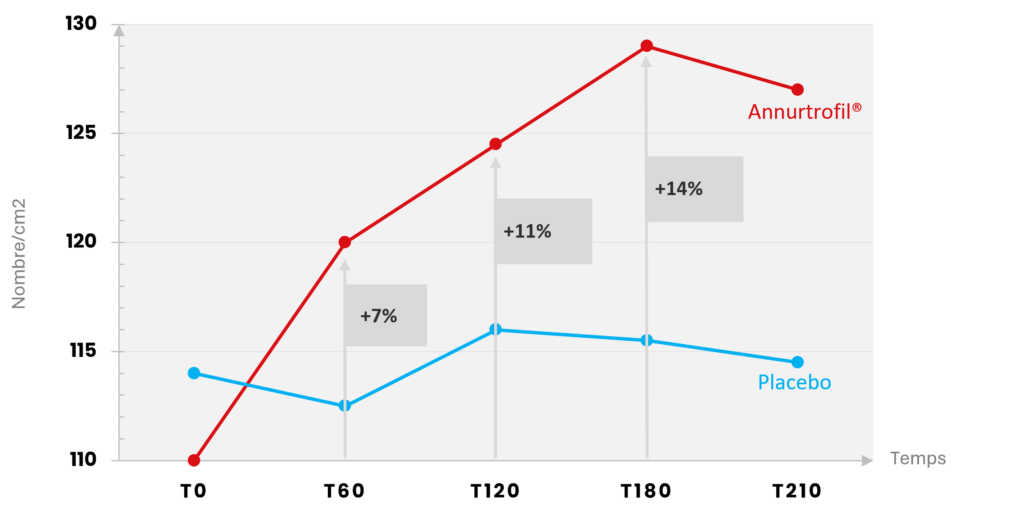
We observed a significant increase in hair density of up to 14% at 180 days in the group supplemented with Annurtrofil® (p<0.05). In contrast, the placebo group showed no significant improvement.
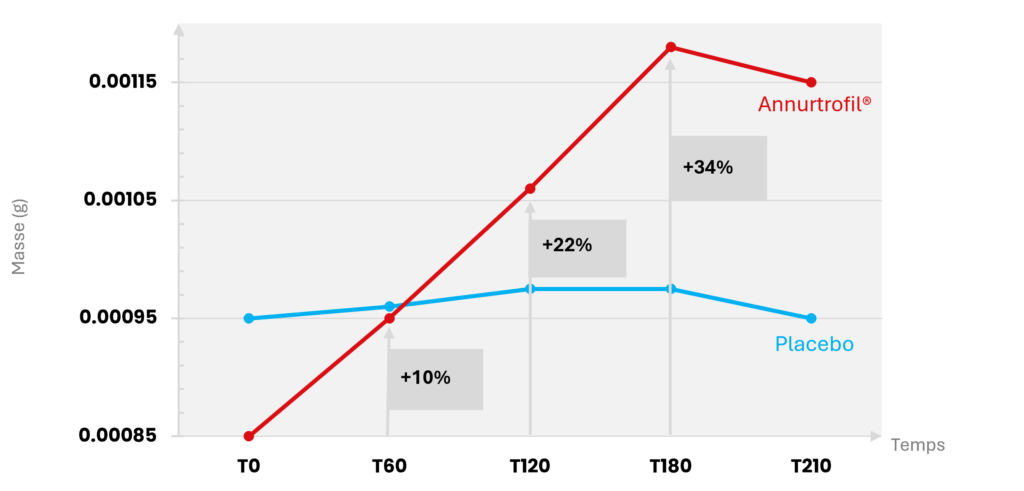
As for hair weight, we observed a significant increase of up to 34% at 180 days in the Annurtrofil® supplemented group (p<0.05). In contrast, the placebo group showed no significant improvement.
Responses to the self-assessment questionnaire on safety and efficacy aspects were very positive:
-
100% positive feedback on product digestibility.
-
83% very positive feedback on hair thickening and density (versus 35% for the placebo group).
-
80% very positive feedback on hair loss reduction (versus 33% for the placebo group).
-
85% very positive feedback on the efficacy of supplementation (versus 28% for the placebo group).