Chondro’Sea©: marine chondroitin sulfate for healthy cartilage.
90% marine chondroitin sulfate. Proven effectiveness on joint pain, joint comfort and daily mobility.

Thank you for your request.
We’ll get back to you as soon as possible!
Continue on page View our catalogWhy choose Chondro’Sea©?

What is Chondro’Sea©?
Chondro’Sea© is derived from fish cartilage, a by-product of fishing in the seas of Southeast Asia. These cartilages are collected from fisheries and processing plants. Chondro’Sea© is a purely marine ingredient, to achieve a chondroitin content of 90% (Ph. Eur. method). Chondroitin sulfate is a molecule naturally present in joint cartilage. It plays a crucial role in the maintenance of cartilage tissue, ensuring its elasticity and hydration. Chondro’Sea© is therefore an ideal ingredient for maintaining healthy cartilage. Its action on joint comfort and improved mobility has been studied with particularly relevant results.
New study and clinical results
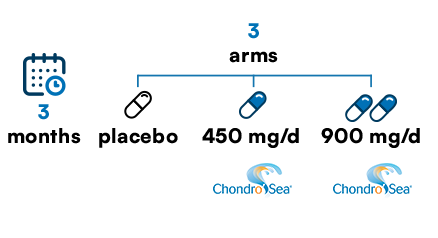
Study design
Chondro’Sea© efficacy on joint pain, articular comfort and daily mobility.
180 volunteers with an average age of 57, suffering from joint pain and functional impairment.
Supplementation
Joint comfort assessment
Joint pain and function
Significant results starting from 450 mg/day !
76% of participants improved
their overall WOMAC score.
The overall WOMAC pain
score decreased by 32%
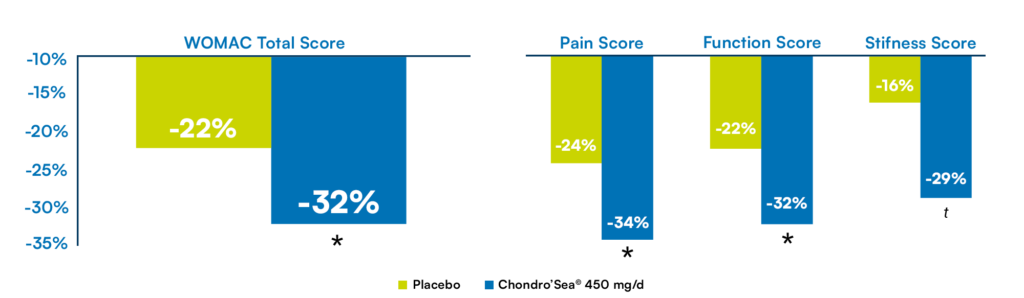
Change from baseline of WOMAC score and sub-scores, after 90 days of supplementation with Chondro’Sea© and Placebo.
Significant difference compared to placebo: * p = 0.05 ; t (trend) : p = 0.1
Mobility
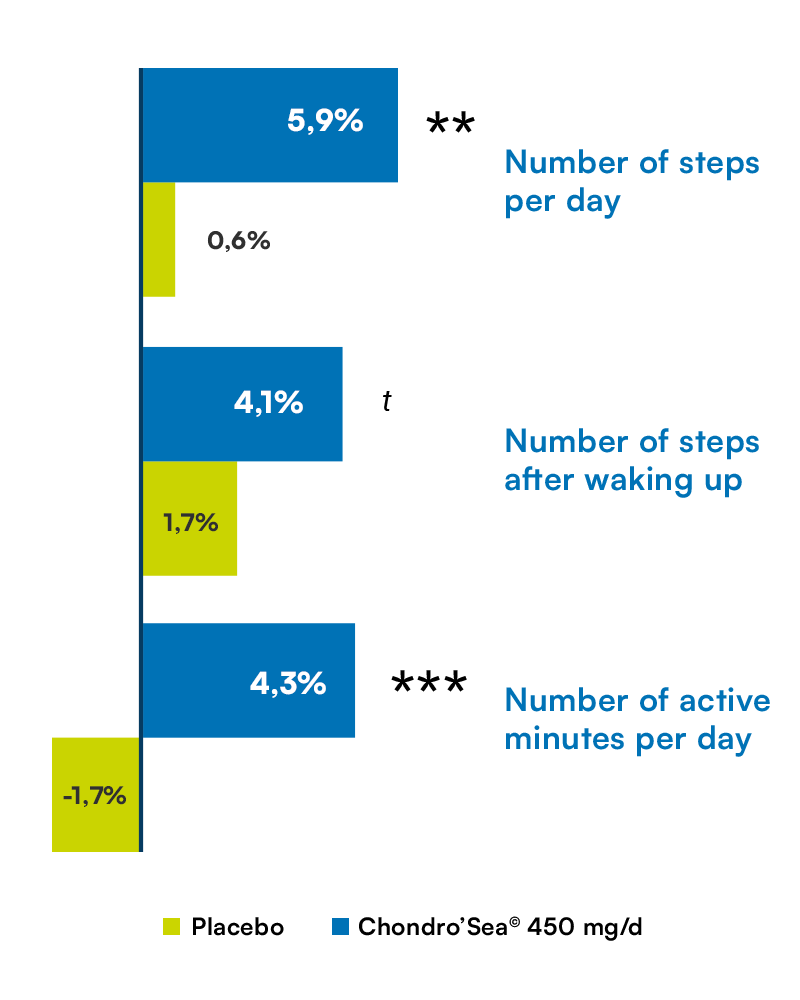
Difference between the first 30 days and the following 60 days.Data collected from the smartwatch.
Significant difference compared to placebo:
***p<0.001 ; **p<0.01 ; t (trend) : p=0.07
Satisfaction
- 73% of participants felt that Chondro’Sea© was effective.
- 72% of participants were satisfied with Chondro’Sea© supplementation.
- 68% of participants felt more fuild in their movements.
- 65% of participants felt more mobile/active than at the start of the study.
Production stages

Quality and safety control.
The entire production process takes place in an ISO22000 and GMP-certified plant, guaranteeing rigorous control of food safety and traceability.
Each batch undergoes in-depth controls to guarantee conformity, authenticity and safety.
We use the Schuster method (HPLC), recognized by the French authorities, to accurately measure chondroitin concentration.
In addition, other controls are carried out to guarantee the marine origin of Chondro’Sea and to verify the absence of chemical or biological contaminants.
Thanks to these strict protocols, we can offer a safe, high-quality product that complies with regulatory requirements.

Regulatory expertise.
We draw up a complete dossier in accordance with the decree of September 26, 2016 establishing the list of substances with nutritional or physiological purposes authorized in food supplements and the conditions of their use.



